
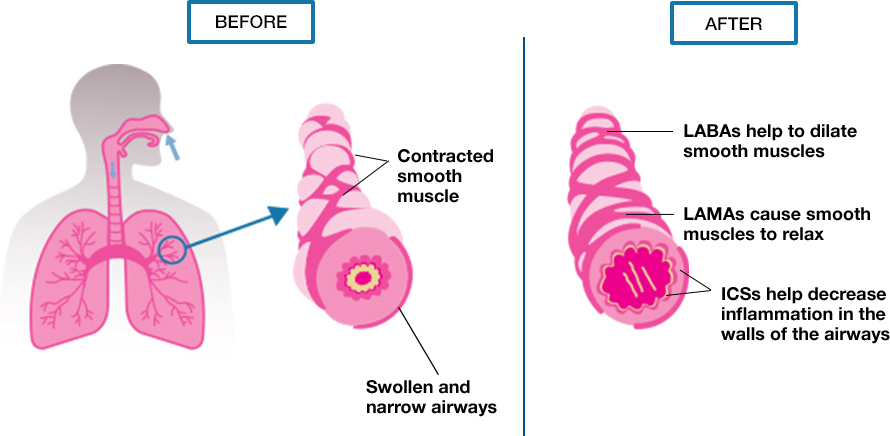
Inhalers are the most common way to take medications for COPD. What is an inhaler, and how does it work? This inflammation causes cough, mucus, and frequent chest infections. The main symptom is breathlessness.Ĭhronic bronchitis: This is damage to the lung airways, again, usually from cigarette smoking. People with COPD typically have two lung problems:Įmphysema: This is damage to the air sacs in the lungs (alveoli), usually from cigarette smoking. Obstructive: This refers to how the airways in the lungs are blocked, meaning that the lungs struggle to move air in and out of the body. To understand how inhalers help COPD, we first need to cover some basics about what happens to the lungs with COPD.ĬOPD stands for “chronic obstructive pulmonary disease.” Let’s break down what that means:Ĭhronic: This means that the disease can’t be cured, so it’s a lifelong condition.
#Trelogy ics laba lama plus#
Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Efficacy and tolerability of budesonide/formoterol added to tiotropium compared with tiotropium alone in patients with severe or very severe COPD: a randomized, multicentre study in East Asia. Efficacy and safety of umeclidinium added to fluticasone furoate/vilanterol in chronic obstructive pulmonary disease: results of two randomized studies. Siler TM, Kerwin E, Sousa AR, Donald A, Ali R, Church A. Comparison of tiotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: a randomized controlled study. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease.
Global Initiative for Chronic Obstructive Lung Disease. The safety and tolerability profile of BUD/GLY/FOR was also found to be comparable to other triple combination therapies.Ĭhronic obstructive pulmonary disease Exacerbations Inhaled corticosteroid Long-acting muscarinic antagonist Long-acting β2-agonist Lung function Network meta-analysis Patient-reported outcomes Safety Triple therapy. In this NMA, BUD/GLY/FOR 320/18/9.6 μg showed comparable efficacy versus other ICS/LAMA/LABA fixed-dose or open combination therapies in terms of reducing exacerbation rates and improving lung function, symptoms and health-related quality of life in patients with moderate-to-very-severe COPD, in line with previously published meta-analysis results of triple combinations in COPD. Sensitivity analyses and meta-regression results for exacerbation outcomes were broadly in line with the base case NMA. Across all outcomes for exacerbations, lung function, symptoms, health-related quality of life, safety, and tolerability, the efficacy and safety of BUD/GLY/FOR were comparable to those of other triple ICS/LAMA/LABA fixed-dose (fluticasone furoate/umeclidinium/vilanterol and beclomethasone dipropionate/glycopyrronium bromide/formoterol fumarate) and open combinations at or over 24 and 52 weeks. LAMA/LABA dual combinations were combined as a single treatment group to create a connected network. Nineteen studies (n = 37,741 patients) met the inclusion criteria of the review 15 contributed to the base case network. Meta-regression and sensitivity analyses were used to assess heterogeneity across studies. Study results were combined using a three-level hierarchical Bayesian NMA model to assess efficacy and safety outcomes at or over 24 and 52 weeks. The methodologic quality and risk of bias of included studies were assessed. A systematic literature review was conducted to identify ≥ 10-week randomized controlled trials, including ≥ 1 fixed-dose or open combination triple-therapy arm, in patients with moderate-to-very severe COPD. This network meta-analysis (NMA) was conducted to compare the relative efficacy, safety, and tolerability of BUD/GLY/FOR 320/18/9.6 µg with other fixed-dose and open combination triple therapies in COPD over 52 weeks, including data from ETHOS. Subsequently, the ETHOS study was published, including data for 8509 patients, assessing the efficacy and safety of BUD/GLY/FOR over 52 weeks. A previous network meta-analysis showed comparable efficacy of the ICS/LAMA/LABA, budesonide/glycopyrronium bromide/formoterol fumarate (BUD/GLY/FOR) 320/18/9.6 µg, to other fixed-dose and open combination triple therapies at 24 weeks in COPD. In patients with chronic obstructive pulmonary disease (COPD) who experience further exacerbations or symptoms, despite being prescribed dual long-acting muscarinic antagonist (LAMA)/long-acting β 2-agonist (LABA) or inhaled corticosteroid (ICS)/LABA therapies, triple ICS/LAMA/LABA therapy is recommended.


 0 kommentar(er)
0 kommentar(er)
